Teeny Cancer-Killing Robots Stalk Tumors
The future of nanorobots, about 10,000 times smaller than a human hair, stalking and killing cancerous tumors.
“We have managed to hide the weapon [DNA origami] in such a way that it can only be exposed in the environment found in and around a solid tumor. This means that we have created a type of nanorobot that can specifically target and kill cancer cells.”
— Björn Högberg, professor Department of Medical Biochemistry and Biophysics at Karolinska Institute
“Malignant growth and normal growth are so genetically intertwined that unbraiding the two is one of the most significant scientific challenges faced by our species.” —Siddhartha Mukherjee, from The Emperor of All Maladies
Teeny robots take on the great killer
One of the ways the marvelous human body keeps itself healthy is by the process of apoptosis (from the Greek for ‘falling off’), whereby infected or damaged cells are broken down and removed by garbage-collecting immune cells.
Except, that is, for cancer cells which can block, evade, or otherwise resist apoptosis. Cancer is cunning. Writer and physician Siddhartha Mukherjee titled his book about cancer The Emperor of All Maladies, writing: “Cancer is built into us: the genes that unmoor normal cell division are not foreign to our bodies but rather mutated, distorted versions of the very genes that allow us to grow, to adapt, to recover, to repair – to live.”
The stakes for finding cancer cures are high…and getting higher. As Dr. Martha Boeckenfeld pointed out in a recent LinkedIn post: “It’s important to note that the global cancer burden is expected to grow significantly in the coming years. Projections indicate that by 2050, there could be over 35 million new cancer cases annually, representing a 77% increase from the 2022 estimates.”
As a way forward, she extols the work of researchers building teeny robots (nanorobots), about 10,000 times smaller than a human hair to stalk and kill cancerous tumors. “The nanorobots carry special proteins that can kill cells. The proteins stay hidden until needed. The robots activate only near cancer cells, where cancer creates an acidic environment, which unlocks the robot’s weapon.”
DNA origami
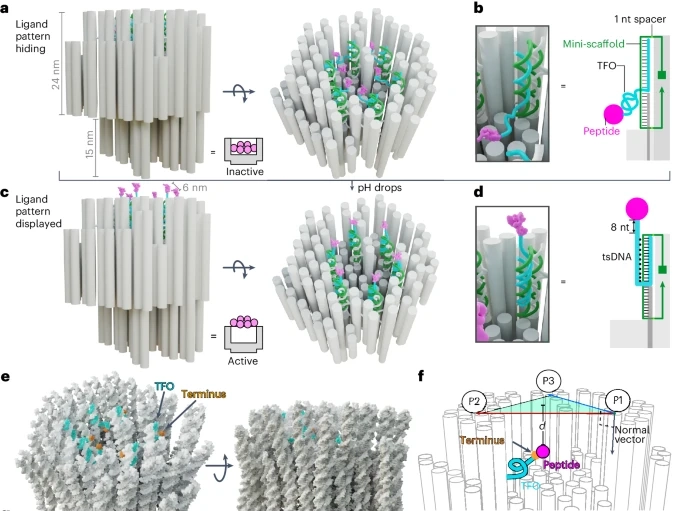
Scientists at Sweden’s Karolinska Institute have pioneered a breakthrough in cancer treatment by developing nanorobots capable of selectively targeting and destroying cancer cells in mice. The team’s innovative approach, which involves the use of “DNA origami” to construct these nanorobots, which was recently published in Nature Nanotechnology. “The nanorobots’ key advantage lies in their ability to spare healthy cells while focusing exclusively on the tumor microenvironment.”
The foundation for this new method comes from the research team’s previous work on designing structures that organize death receptors on the surface of cells, triggering programmed cell death. By arranging peptides in a hexagonal pattern, these structures can induce cell death. However, delivering such a system to the body in a way that avoids harming healthy tissues was a challenge.
Lead researcher Professor Björn Högberg from the Department of Medical Biochemistry and Biophysics at Karolinska Institute explained how the problem was solved by enclosing the death-inducing mechanism within a DNA-built nanostructure. This allows the nanorobot to remain inactive until it encounters a specific environment, typically found around tumors, where it can then release its payload.
The team’s strategy centers on exploiting the acidic environment common to tumors. Tumor cells often reside in areas with a lower pH, around 6.5, while normal cells exist in neutral pH environments. The researchers designed the nanorobots so that the toxic peptides they carry remain hidden in normal pH conditions but are activated when the pH drops to tumor levels. Once activated, these peptides bind to cancer cell receptors, triggering apoptosis, or programmed cell death.
This mechanism was tested on mice with breast cancer tumors. The results were impressive: the nanorobots reduced tumor growth by 70% compared to those treated with an inactive version of the robot. While these results offer considerable promise, Yang Wang, the study’s first author and researcher at Karolinska, emphasized the need for further testing. The researchers aim to explore how the nanorobots perform in more advanced cancer models and what side effects they might cause.
The potential for this technology to be refined further is exciting. The researchers are considering ways to enhance the nanorobots’ targeting capabilities, perhaps by attaching proteins or peptides that bind specifically to different types of cancer cells. If successful, this could revolutionize how cancer is treated, offering a more precise and less harmful alternative to current methods such as chemotherapy, which often causes damage to healthy tissues.
Nanotechnology, particularly the use of DNA origami to create nanorobots, opens up vast possibilities in medicine. The origami technique allows scientists to manipulate DNA into intricate three-dimensional shapes, creating nanoscale devices capable of performing complex functions. In this case, it enables the nanorobots to carry and deliver cancer-killing agents only when needed.
One of the key advantages of this method is the nanorobots’ biocompatibility. Built from DNA, they can travel through the body without causing immune reactions. As they circulate, these nanorobots are designed to detect the acidic pH surrounding cancer cells, where they unleash their lethal payload. This precise targeting minimizes collateral damage to healthy cells, a significant improvement over traditional cancer treatments.
The success of the DNA origami nanorobots in reducing tumor growth in mice is a promising first step toward potential human applications. If this technology proves effective in humans, it could lead to more targeted and less invasive cancer treatments, significantly improving outcomes for patients while reducing the harsh side effects associated with current therapies.
This innovation also holds potential beyond cancer treatment. The principles behind DNA origami nanorobots could be adapted for other diseases, such as autoimmune disorders or viral infections, where targeted cell death might be beneficial. The programmable nature of DNA origami offers scientists a powerful tool for designing highly specific treatments.
However, there are still hurdles to overcome before nanorobot technology becomes a standard cancer therapy. Researchers will need to ensure that the nanorobots remain stable and effective in the human body over time. Additionally, scaling up production for clinical use poses challenges. Thorough testing is also needed to rule out any unintended side effects.
As Björn Högberg noted, one of the primary concerns is making sure that the nanorobots do not cause harm to healthy cells. The fact that the nanorobots are inactive under normal physiological conditions provides some reassurance, but long-term safety studies will be essential before moving forward with clinical trials.
Yang Wang, co-author of the study, echoed this sentiment, noting that the team is focused on refining the technology to make it even more selective and efficient. Researchers are exploring the possibility of adding surface markers to the nanorobots that would allow them to bind only to certain cancer types, enhancing their specificity.
The development of DNA origami nanorobots marks a major step forward in oncology. If the technology continues to progress and proves effective in human trials, it could transform the way cancer is treated. Instead of relying on broad-spectrum therapies like chemotherapy, doctors might one day use highly targeted nanobots to destroy cancer cells while leaving healthy tissue untouched.
This emerging field of nanomedicine is generating excitement among scientists and offering hope to millions of people affected by cancer. While challenges remain, the success of these nanorobots in animal models provides a glimpse of a future where cancer is treated with unprecedented precision and effectiveness.
